
PREIMPLANTATION GENETIC TESTING FOR CHROMOSOMAL ANEUPLOIDY (PGT-A)

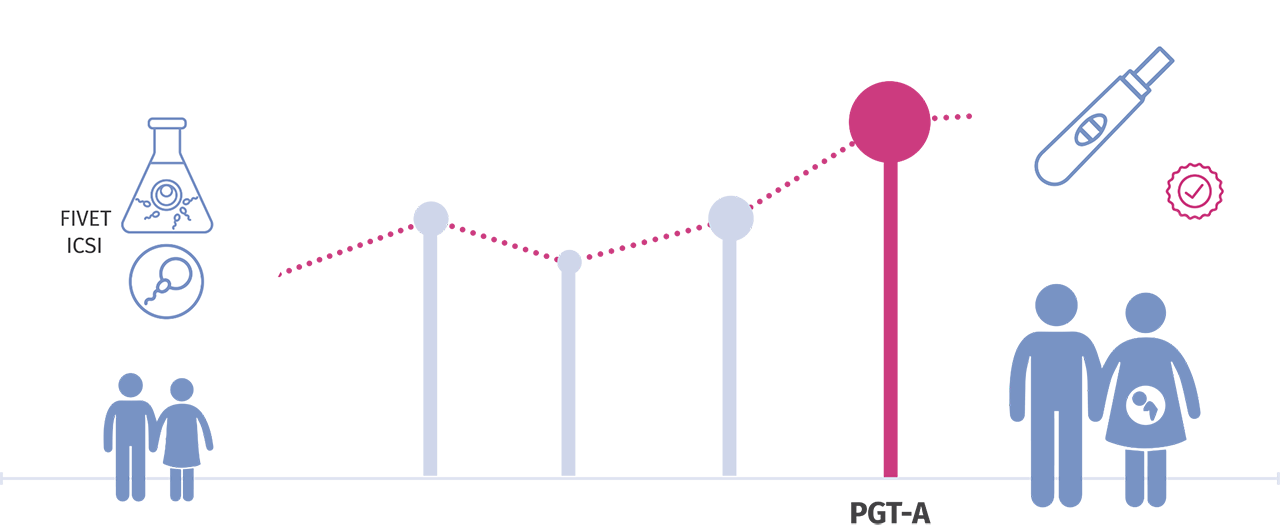
Preimplantation genetic testing for chromosomal aneuploidy (PGT-A) is a technique used in conjunction with In-Vitro fertilization (IVF) to detect embryos with extra or missing chromosomes (aneuploidy).
Through PGT-A, the selection of embryos to be transferred to the uterus is based not only on a morphological evaluation but also on the related chromosomal ploidy, which reflects their possibility of giving rise to an ongoing pregnancy.
Embryos that are affected by certain chromosomal conditions can lead to failure of implantation, pregnancy loss, or result in the birth of a child with physical and/or mental problems. The purpose of PGT-A is to help prevent adverse outcomes by identifying affected embryos in the laboratory and preventing them from being transferred into the uterus.
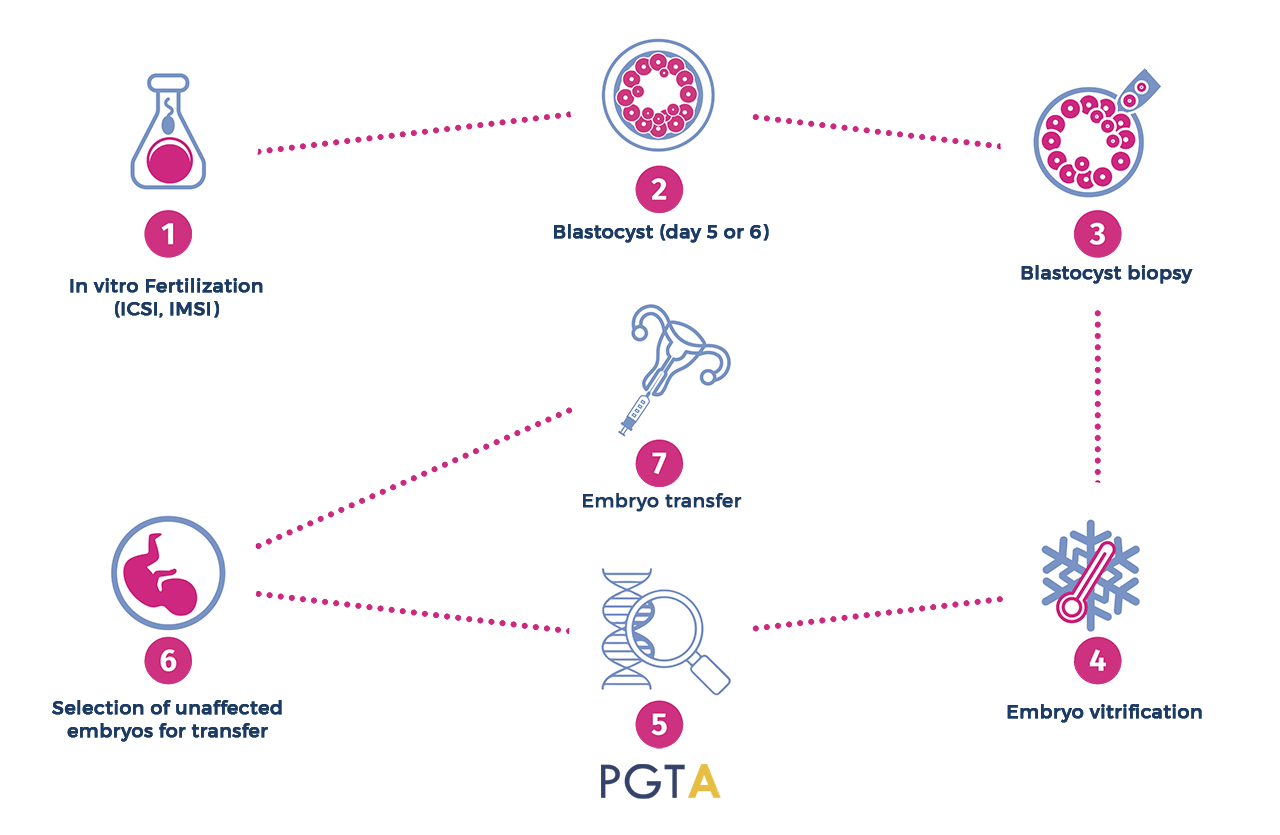
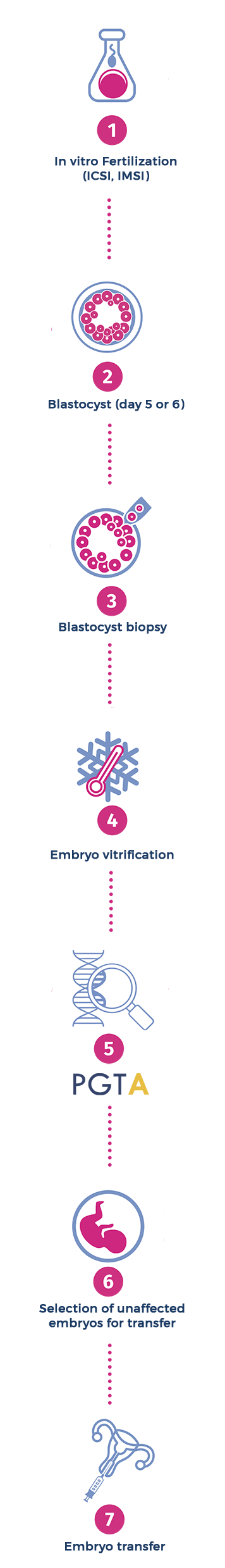
EMBRYO BIOPSY

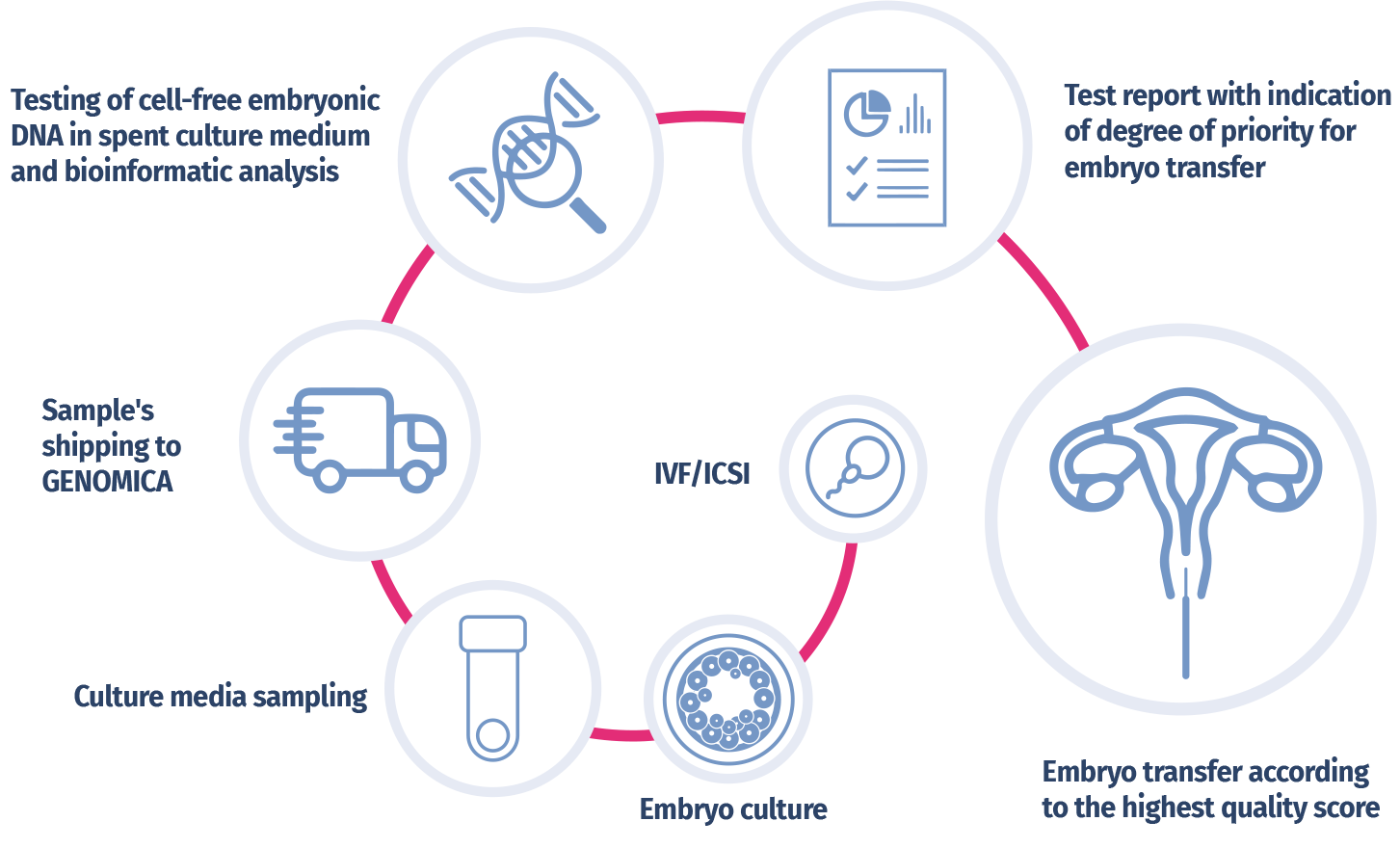
PGT-A usually requires that the couple undergoes to an in vitro fertilization (IVF) treatment (1). After five or six days from fertilization, the embryos usually consist of a tiny ball of a couple hundred cells (blastomeres), known as blastocyst (2). To test the blastocyst, an opening is made in the covering of the embryo (zona pellucida), through the action of a laser beam. Blastomeres are then removed (biopsied) from trophectoderm of each embryo (3) and subjected to aneuploidy screening (5). Then, all embryos will be frozen (4) and will remain at the IVF center for a future frozen embryo transfer. Embryos that will result to be euploid (absence of aneuploidy) (6) on chromosomal analysis will be transferred to the womb (7), ultimately producing unaffected babies. The embryo biopsy procedure consists in making a small perforation in the zona pellucida (the wall that surrounds the embryo) through the action of a laser beam. Although this procedure is performed by expert embryologists, the manipulation of the embryo is still invasive and the risk (albeit low) of damaging the embryo during the biopsy cannot be excluded.
CELL-FREE EMBRYONIC DNA IN THE CULTURE MEDIUM

The recent discovery of cell-free embryonic DNA in spent culture medium has opened
new perspectives in the IVF field, in particular as regards the non-invasive embryo
aneuploidy testing.
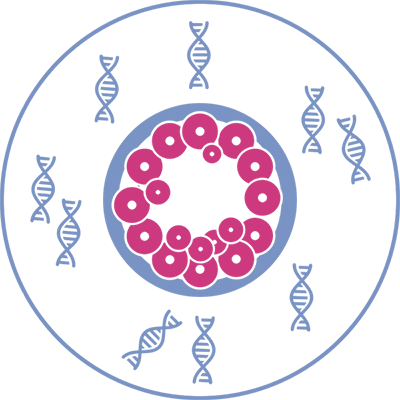
IVF derived embryos, during their in-vitro development, naturally release into the culture medium DNA fragments,
named embryonic DNA, with higher concentrations as the number of cells increases with the embryo development
at blastocyst stage. This feature allows, through the use of state-of-the-art instrumental technologies, to assess the
embryonic chromosome copy number in a non-invasive manner, without the need to perform the trophectoderm
biopsy.
ABOUT THE TEST


EmbryoAdvance is a pioneering non-invasive pre-implantation genetic test developed by GENOMICA that allows identification of embryos with a higher probability of euploidy, and therefore with a higher implantation potential, by analyzing the cell-free embryonic DNA in spent culture media.
HOW DOES THE TEST WORK?

EmbryoAdvance assigns the embryos a degree of priority for transfer to the uterus, based on information on the chromosome copy number of the embryos. This information may be used for selecting optimal embryo to prioritize and transfer first in an IVF cycle, thus maximizing the chances of success of the IVF treatments.
WHO CAN BENEFIT FROM THE TEST?

Any patients who wish to increase their chances of pregnancy, without performing embryo biopsy.
BENEFITS


It has the potential to increase the effectiveness of IVF techniques in groups of patients characterized by reduced reproductive performance, in which conventional technologies have not been successful.
Improvement in the success rates of IVF treatments and reduction of the time to pregnancy.

Reduction of the risk of spontaneous miscarriages,
depending on the possible presence of chromosomal abnormalities in the embryo.

It is a non-invasive and safe test.
It does not require manipulation of the embryo.
No need for embryo biopsy.
It reduces the cost of the clinic by making more affordable chromosomal assessment of embryos in IVF treatments.
NON-INVASIVE PGT-A PROCEDURE

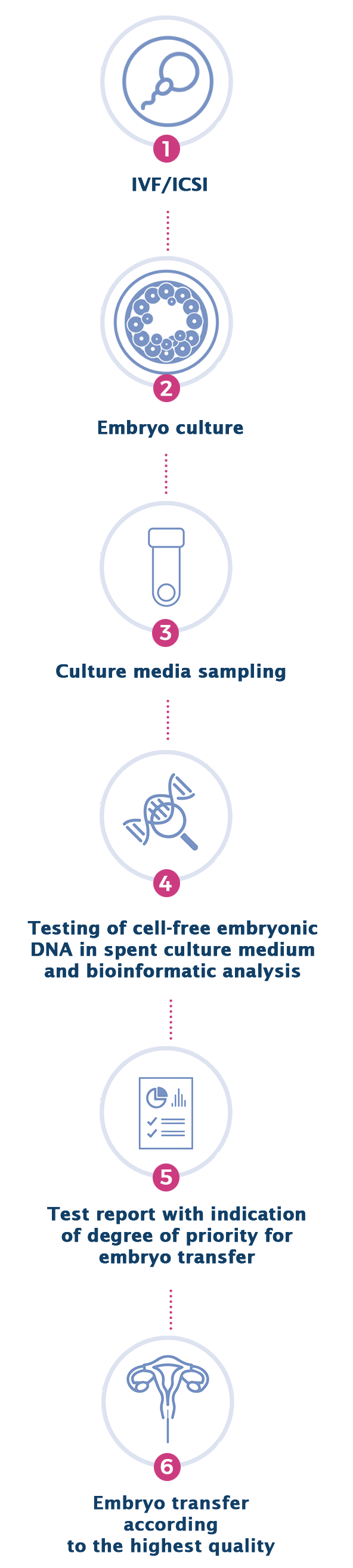
The EmbryoAdvance test analyzes the cell-free embryonic DNA released into the culture medium during the development of the embryo, using Next Generation Sequencing (NGS) technologies and advanced bioinformatic analyses. The test is performed by collecting a sample of embryo culture medium by the embryologists of the IVF center, carried out on day 6 or day 7 of development. Subsequently, the chromosomal regions of embryonic DNA are sequenced using NGS sequencers. The chromosomal sequences are then quantified through an advanced bioinformatic analysis, in order to detect embryonic chromosomal aneuploidies, identified by a greater amount of embryonic sequences relating to a specific chromosome as compared to a "normal" reference standard. A proprietary algorithm will allow to obtain a quality score for each embryo and therefore a degree of priority (High, Medium, Low) for its transfer to the uterus.
TEST RESULTS

HIGH SCORE:
High Priority: this test result
indicates that the test has identified
embryos with a high probability of
euploidy and, therefore, with higher
implantation potential.
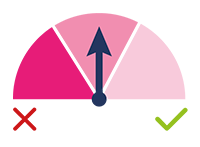
MEDIUM SCORE:
Intermediate Priority: this test result
indicates that the test has identified
embryos with an intermediate
probability of aneuploidy and,
therefore, with reduced implantation
potential or higher risk of abortion.
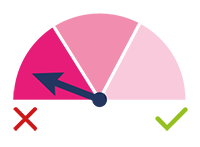
LOW SCORE:
Low Priority: this test result
indicates that the test has identified
embryos with a high probability of
aneuploidy and, therefore, with very
low implantation potential or with a
high risk of miscarriage.
On rare occasions, the test may produce suboptimal or inconclusive results. In such cases, additional genetic assessments may be required, which might include an embryo biopsy

Turnaround time

WHY CHOOSE


ADVANCED MOLECULAR DIAGNOSTICS SOLUTIONS USING STATE-OF-THE ART TECHNOLOGIES
GENOMICA is recognized as one of the most advanced molecular diagnostics laboratory in Europe, both for the state-of-the-art instruments and technologies, as well as for its high quality standards. With a comprehensive portfolio of over 10.000 genetic tests, GENOMICA is able to satisfy increasingly specialised requests in the field of molecular genetics, providing physicians and their patients with innovative and highly specialised diagnostic solutions for any clinical need

Over 100.000
genetic tests/year

Test performed in Italy
(Rome or Milan)

20+ years experience in prenatal molecular diagnostics

Fast
TAT

Dedicated
R&D team

Laboratories with groundbreaking technologies and high quality standards

Personalized genetic counseling with genetic counselors experts in discussing genetic test results and familial risks

International
Partnership
WHY CHOOSE


ADVANCED MOLECULAR DIAGNOSTICS SOLUTIONS USING STATE-OF-THE ART TECHNOLOGIES
GENOMICA is recognized as one of the most advanced molecular diagnostics laboratory in Europe, both for the state-of-the-art instruments and technologies, as well as for its high quality standards. With a comprehensive portfolio of over 10.000 genetic tests, GENOMICA is able to satisfy increasingly specialised requests in the field of molecular genetics, providing physicians and their patients with innovative and highly specialised diagnostic solutions for any clinical need

Over 100.000 genetic tests/year

Test performed in Italy
(Rome or Milan)

20+ years experience
in prenatal molecular diagnostics

Fast
TAT

Dedicated
R&D team

Laboratories with groundbreaking technologies and high quality standards

Personalized genetic counseling with genetic counselors experts in discussing genetic test results and familial risks

International
Partnership
Information request for EMBRYOADVANCE Test

Fill out this form for a free consultation.
One of our professionals will contact you, free of charge and without obligation, to provide you with all the information you need.


 Italiano
Italiano English
English